Combustion Theory
What is Combustion?
- High speed fuel oxidization that produces light, heat and flame
- Three necessary components: oxygen, ignition, fuel
- If one of the components is not present, combustion does not occur
- Removal of either fuel or air will stop combustion
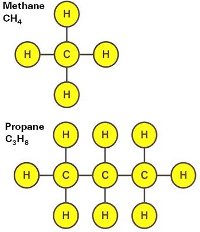
Gaseous Fuel Composition
- Hydrocarbons determine calorific value
- More carbon content means more heating value
A hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. A molecule of propane and methane is pictured on the left.
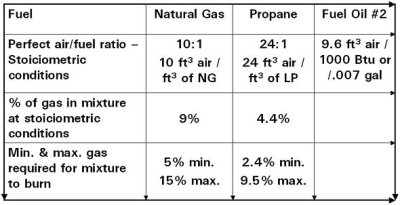
Fuel to Air Mixture
- Hydrocarbons determine calorific value
- More carbon content means more heating value
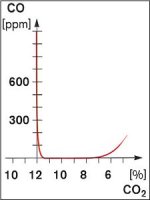
Fuel to Air Ratio
- There are two ways of creating CO with the wrong fuel/air ratio
- Insufficient air (sub-stoichiometric)
- Too much air (over-airing)
- Sub-stoichiometric conditions create CO very quickly
- CO production with over-airing is less rapid
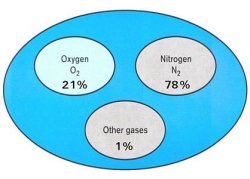
Air is Composed of:
- 21% Oxygen
- 78% Nitrogen
- 1% Carbon Dioxide and other gasses (argon, krypton, etc.)
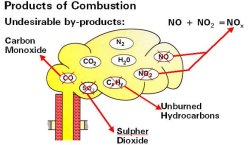
Combustion of Natural Gas
In simple terms, the chemical equation of natural gas is:
AIR + NATURAL GAS -> HEAT + WATER VAPOR + CO2
However, since the oxygen in real-world combustion comes from the air around us, undesirable byproducts in the form of nitrogen will be produced.
Perfect (Stoichiometric) Combustion
To mimimize the amount of 'undesirable' emissions, a perfect air to fuel ratio has to be achieved.
When theoretical perfect air/fuel mixtures are applied, stoichiometric combustion is achieved.
Eg: 10 parts air to 1 part natural gas
Each fuel has a maximum CO2 percentage when stoichiometric combustion is achieved
- Natural gas: 11.8 - 12.5% CO2
- Propane gas: 13.6 - 13.8% CO2
- Fuel oil: 15.4 - 15.6% CO2
Real World Combustion
In the real world, it is very difficult to maintain the perfect 10:1 air/gas ratio due to a number of factors.
- Air Temperature Changes
- Air Pressure Changes
- Varying Furnace Pressures
- Varying Draft Pressure
- Fan Contamination
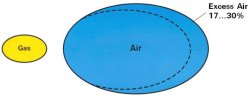
Excess Air
The common way of expressing the usage of more than the stoichiometric combustion air is percent excess combustion air.
-Too little excess air creates CO and/or soot
-Too much excess air is a waste of energy
-For safety, the burner is usually adjusted for an additional 17-30% excess air
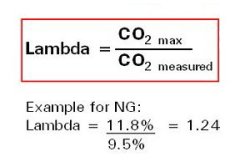
Measuring Excess Air
Excess air is measured by analyzing flue gas (exhaust gas)
Excess air is expressed as Lambda
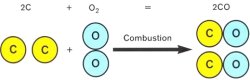
Carbon Monoxide
Incomplete combustion causes single carbon atoms to combine with single oxygen atoms, productin carbon monoxide.
Reasons why CO is formed:
- Insufficient air
- Insufficient heat (chilled flame)
- Too much air
CO is a an odourless, colourless gas
CO is undetectable without instruments such as a flue-gas analyzer
CO is a flammable gas that will ignite in the range of 12.5 to 74% CO to air
CO is created when the fuel/air ratio is not set correctly
Effects of Exposure to Carbon Monoxide
- 12,800 ppm - Death within 1 to 3 minutes.
- 1,600 ppm - Nausea within 20 minutes. Death within one hour.
- 800 ppm - Nausea and convulsions. Death within two hours.
- 400 ppm - Frontal headaches 1-2 hours. Life threatening after 3 hours.
- 50 ppm - Maximum concentrations for continuous exposure in any eight-hour period.
- 9 ppm - Maximum indoor air quality level.
- 0 ppm - Desirable level.
Oil Combustion
- Oil contains considerably more nitrogen than gaseous fuels
- NOX levels are generally much higher than on natural gas
- Oil flue gasses are tested with a Bacharach smoke spot test and combustion analyzer
- Test gives a number from 0 (no smoke) to 10